TRINTELLIX® (vortioxetine) is indicated for the treatment of Major Depressive Disorder (MDD) in adults.

Patient portrayal.
Individual results may vary.
Clinical Data
What might the role of efficacy be in the next chapter?
TRINTELLIX MONOTHERAPY RELIEVED THE OVERALL SYMPTOMS OF MDD1
Based on 6 short-term clinical studies that1:
- Were randomized, placebo-controlled, and double-blind, and lasted 6 to 8 weeks
- Gave adult patients 5 to 20 mg of TRINTELLIX once daily
- Primary endpoint: improvement in the total score of MADRS or HAM-D24
- Concluded that at least 1 of the studied doses of TRINTELLIX in each short-term clinical study was superior to placebo as measured by the primary efficacy endpoint
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo in 6- to 8-week studies) were nausea, constipation, and vomiting.1
Two additional US studies (not shown in the table below) failed to show effectiveness at the 5-mg dose.1
Studies 1-6: short-term efficacy*

*Adult patients aged 18-75 years in 5 studies and aged 64-88 years in 1 elderly study.1
†Elderly patients can be at greater risk for hyponatremia.1
‡Doses significantly superior to placebo after adjusting for multiplicity.1
HAM-D24, Hamilton Depression 24-item Rating Scale; LOCF, last observation carried forward; MADRS, Montgomery‑Åsberg Depression Rating Scale; MMRM, mixed model repeated measures.
More than 2300 patients were enrolled across these 6 clinical trials1

Indiana University, Parkview Physicians Group Mind-Body Medicine, Fort Wayne, Indiana
"The short-term efficacy data for TRINTELLIX demonstrated superiority over placebo in managing the overall symptoms of MDD across a range of dosages in adult and elderly patients.1"
Jay D. Fawver, MD, is the medical director at Parkview Mind‑Body Medicine in Fort Wayne, IN. At Mind‑Body Medicine, he orchestrates the treatment of over 8000 current patients in a shared pharmacological treatment model. He works with 10 nurse practitioners, assisted by nurses and medical assistants, utilizing customized electronic medical records to coordinate consistent clinical care with personalized and precision treatment strategies. His nurse practitioners provide integrated treatment by having access to direct consultation with Dr Fawver and the other nurse practitioners while utilizing treatment algorithms for various conditions and genetic profiles. Patients are monitored for treatment response and recovery utilizing patient-rated outcome metrics for mood, anxiety, cognition, insomnia, daily functioning, and side effects.
Since March 2020, Mind-Body Medicine has initiated telehealth for a majority of outpatients utilizing the new Epic Video Client internet platform. Utilizing this platform from their smartphones, tablets, or laptops, patients are prompted to complete outcome questionnaires on their MyChart portals 1 to 2 days prior to each appointment. These outcome metrics include the PHQ-9, GAD-7, Perceived Deficits Questionnaire (PDQ-20), Insomnia Severity Index (ISI), Work and Social Adjustment Scale (WSAS), and Patient Rated Inventory of Side Effects (PRISE), guiding treatment decisions and enhancing the efficiency of the outpatient video visit. Dr Fawver is a clinical professor of psychiatry at the Indiana University School of Medicine and is board certified by the American Board of Psychiatry and Neurology. He has been named a Distinguished Life Fellow of the American Psychiatric Association. A graduate of Purdue University School of Pharmacy, Dr Fawver was a pharmacist prior to his medical training. He has been an advisor to several pharmaceutical companies for over 30 years, participating as a principal investigator and assisting in the development of medication studies and outcomes of disease states such as depression, bipolar disorder, schizophrenia, and fibromyalgia with more recent work involving genetic correlations with medication treatment outcomes.
Since October 2019, Dr Fawver has been a certified Epic Physician Builder where he designs computer programs for charting in the electronic medical records based upon best practice guidelines.
Since 1997, he has been the host and producer of the Public Broadcasting System’s Matters of the Mind with Dr Jay Fawver, a weekly 30-minute live call-in television program addressing mental health topics.
Dr Fawver was paid as a consultant for Takeda and Lundbeck.

Gregory Mattingly, MD
Washington University, Midwest Research Group, St. Charles, Missouri
"TRINTELLIX was evaluated for improvement in MADRS and HAM‑D24 total scores across 6 randomized, placebo-controlled, double-blind 6- to 8-week studies at dosages ranging from 5 mg to 20 mg once daily. In each study, at least one dose of TRINTELLIX was superior to the placebo.1-7 This makes me confident prescribing TRINTELLIX to appropriate patients with MDD."
Dr Mattingly is a physician and principal investigator in clinical trials for Midwest Research Group. He is also a founding partner of St. Charles Psychiatric Associates, where he treats adults, adolescents, and children with ADHD. Dr Greg Mattingly is board-certified in adult and adolescent psychiatry and has been a principal investigator in over 300 clinical trials focusing on ADHD and related conditions.
Having served on numerous national and international advisory panels, Dr Mattingly has received awards and distinctions for clinical leadership and neuroscience research. He currently serves on the board of directors for the American Professional Society of ADHD and Related Disorders (APSARD).
Dr Mattingly was paid as a consultant for Takeda and Lundbeck.
IMPROVED MDD PATIENTS' SPEED OF PROCESSING, WHICH MAY BE IMPAIRED IN MDD1
What is speed of processing?1,8
- It can be described as the pace at which a person can accurately process information8
- Speed of processing is an aspect of cognitive function1
In two 8-week, randomized, double-blind, placebo-controlled studies (dosed once daily), TRINTELLIX significantly improved performance on the DSST in adults with acute MDD.1
Studies 7 and 8: improvement in speed of processing during the treatment of MDD1
Endpoint Measured: Number of Correct Responses on the DSST1
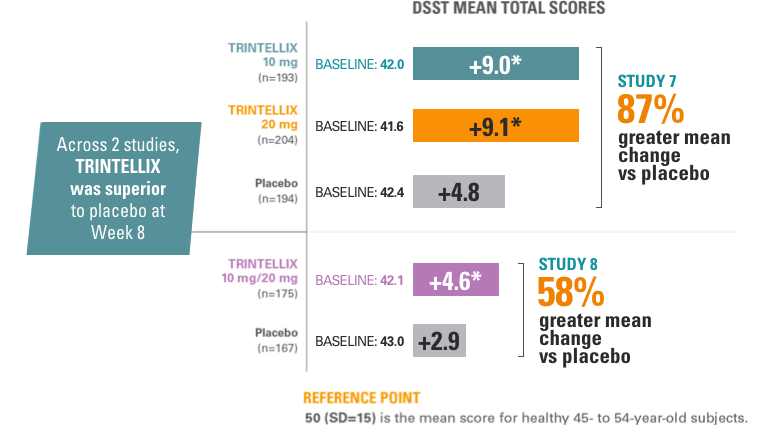
*Doses are statistically significantly superior to placebo: P<0.001 for Study 7 and P<0.05 for Study 8.1,5,9,11
Abbreviations: DSST, Digit Symbol Substitution Test; SD, standard deviation.
About the DSST and speed of processing
- The DSST is a neuropsychological test that most specifically measures speed of processing, an aspect of cognitive function that may be impaired in MDD.1
The effects observed on DSST may reflect improvement in depression. Comparative studies have not been conducted to demonstrate a therapeutic advantage over other antidepressants on the DSST.1
ONLY TRINTELLIX offers data on speed of processing during the treatment of MDD in its US Prescribing Information.13
- Common AEs (incidence ≥5% for TRINTELLIX) in Study 7 were nausea (16.4%, 20.8%, 4.1%) and headache (8.2%, 12.6%, 7.1%) for TRINTELLIX 10 mg/day, and TRINTELLIX 20 mg/day, and placebo, respectively.9 SAEs were reported by two patients in the TRINTELLIX 20 mg group and two patients in the placebo group. Withdrawals due to TEAEs were 2.6% (TRINTELLIX 10 mg), 4.3% (TRINTELLIX 20 mg), and 4.1% (placebo).
- Common AEs (incidence ≥5% for TRINTELLIX) in Study 8 were nausea (20.4%, 4.2%), headache (10.2%, 8.4%), and diarrhea (5.6%, 2.6%) for TRINTELLIX and placebo, respectively.10 Withdrawals due to TEAEs were 3.6% (TRINTELLIX 10 mg/20 mg) and 3.7% (placebo). One patient in the TRINTELLIX group attempted suicide, and one patient in the placebo group was hospitalized for worsening of depression.
TRINTELLIX has been studied in over 5,800 adults
with MDD between the ages of 18 and 88.1

Brooke Kempf, PMHNP-BC
Psychiatric Mental Health Nurse Practitioner Hamilton Center, Terre Haute, Indiana
"Speed of processing, which may be impaired in MDD, can be described as how quickly a person can accurately process information.
The effects observed on DSST may reflect improvement in depression. Comparative studies have not been conducted to demonstrate a therapeutic advantage over other antidepressants on the DSST."
Ms. Kempf began working at Hamilton Center, Terre Haute, Indiana in May 1995 as a Registered Nurse. She briefly left the organization in 2001 to continue her work as a Registered Nurse in the Behavioral Health Unit of Regional Hospital, but returned in 2002 where she works with patients with serious mental illnesses in multiple settings throughout the organization. In 2010, after receiving her Masters, she was promoted to a Psychiatric Nurse Practitioner where she works under Hamilton Center’s Chief Medical Officer to provide patient evaluations, diagnosis, and medication management for consumers with various serious mental illnesses. In addition, Ms. Kempf is an adjunct faculty member and Clinical Coordinator for IUPUI’s Psychiatric Mental Health Nurse Practitioner program. Ms. Kempf also enjoys providing education to other providers as a speaker for Takeda & Lundbeck, Alkermes, Otsuka, and Allergan Pharmaceuticals.
Ms. Kempf was paid as a consultant for Takeda and Lundbeck.
In long-term studies, TRINTELLIX monotherapy reduced the risk of recurrence of a depressive episode1
Study 10 (US-based)
Primary endpoint: risk of recurrence† during the first 28 weeks of the double‑blind phase
Randomized, double-blind, placebo-controlled, 48-week, 2-phase study in adult patients with MDD aged 18 to 75 years.1,11 In the initial, open-label phase, all patients (n=1106) were treated with TRINTELLIX 10 mg/day for 16 weeks. Patients who met remission criteria* after 16-week, open‑label treatment phase of TRINTELLIX 10 mg/day were randomized into a double-blind phase 1:1:1:1 ratio to TRINTELLIX 5 mg/day, 10 mg/day, 20 mg/day, or placebo for 32 weeks.
Study 10: risk of recurrence after remission1,11
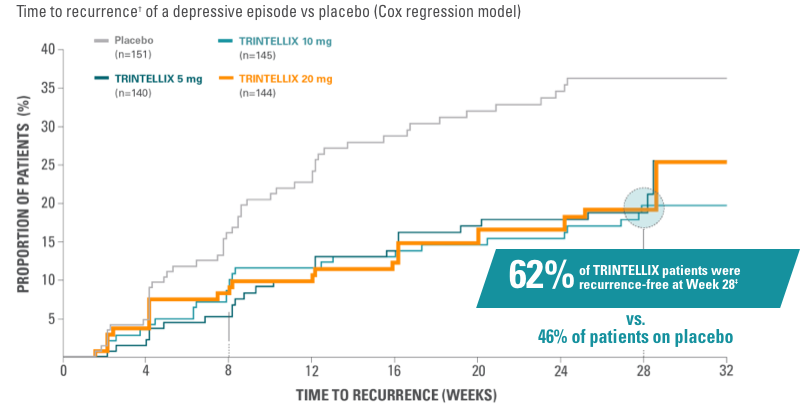
*Remission was defined as MADRS total score ≤12 at both Weeks 14 and 16.1
†Recurrence of a depressive episode was defined as MADRS total score ≥22 or lack of efficacy as judged by the investigator.1
‡Percentage of patients with recurrence and percentage of patients recurrence-free at Week 28 were each divided by the total number of patients in study.11
Primary endpoint: Risk of recurrence† of a depressive episode was significantly greater for patients on placebo (n=151) vs TRINTELLIX (n=429) during the first 28 weeks of the double-blind phase (5 mg: HR, 1.9; 95% CI, 1.2-3.1; P=0.006. 10 mg: HR, 2.1; 95% CI, 1.3-3.4; P=0.002. 20 mg: HR, 2.1; 95% CI, 1.3-3.4: P=0.003).1,11 Significantly fewer patients taking TRINTELLIX experienced recurrence (19% [n=27], 18% [n=26], and 17% [n=25] for 5 mg, 10 mg, 20 mg, respectively; vs 33% [n=49] taking placebo, P<0.05).
Study 10: long-term, 48-week study11

Abbreviations: AEs, adverse events; CI, confidence interval; HR, hazard ratio; MDE, major depressive episode; SAEs, serious adverse events; TEAEs, treatment-emergent adverse events.
Open-label phase
- 9 SAEs; only TRINTELLIX-related SAE was active suicidal ideation with a plan; 1 SAE was passive suicidal ideation11
Double-blind phase
- 9 SAEs; none deemed related to TRINTELLIX; 1 SAE was a completed suicide11
IMPORTANT NOTE ABOUT MAINTENANCE STUDIES: The TRINTELLIX maintenance studies, Studies 9 and 10 in the Prescribing Information, are not comparable.1,11,14 Study 9 was conducted only ex-US while Study 10 was conducted in the US. In addition, patients in Study 10 were required to have been experiencing their current MDE longer, and to have had more previous MDEs. Also, lengths of the open-label and double-blind phases, dosing in each of these phases, criteria for randomization to the double-blind period, and primary endpoints differed between the studies. Each study should be considered independently.

Gregory Mattingly, MD
Washington University, Midwest Research Group, St. Charles, Missouri
"Data from 2 randomized long‑term studies demonstrated TRINTELLIX maintained efficacy in patients aged 18 to 75. In each study, patients who achieved remission during the open-label phase were randomized to TRINTELLIX or placebo in the double-blind phase.1 In both studies, patients randomized to TRINTELLIX were at a significantly less risk of recurrence in the double-blind phase,1,2 demonstrating that TRINTELLIX helped these patients reduce their risk of recurrence of depressive episodes."
Dr Mattingly is a physician and principal investigator in clinical trials for Midwest Research Group. He is also a founding partner of St. Charles Psychiatric Associates, where he treats adults, adolescents, and children with ADHD. Dr Greg Mattingly is board-certified in adult and adolescent psychiatry and has been a principal investigator in over 300 clinical trials focusing on ADHD and related conditions.
Having served on numerous national and international advisory panels, Dr Mattingly has received awards and distinctions for clinical leadership and neuroscience research. He currently serves on the board of directors for the American Professional Society of ADHD and Related Disorders (APSARD).
Dr Mattingly was paid as a consultant for Takeda and Lundbeck.
Study 09 (Non-US)1,14
Primary endpoint: time to recurrence within the first 24 weeks of the double‑blind period
Randomized, double-blind, placebo‑controlled 2-phase study up to 64 weeks in adult patients with MDD aged 18 to 75 years. In the initial, open‑label phase, all patients (n=639) received flexible doses of TRINTELLIX (5 mg or 10 mg)* once daily for 12 weeks. Patients who met remission criteria‡ after 12‑week open-label phase of TRINTELLIX 5 mg/day or 10 mg/day were randomized to double-blind phase to continue final fixed dose or placebo.§
Study 9: long-term efficacy
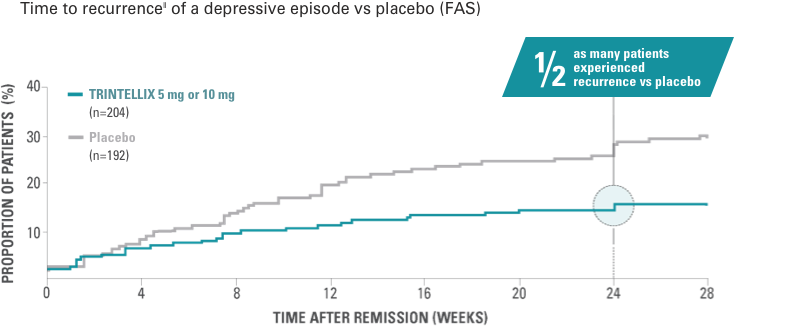
Primary endpoint: Risk of recurrence|| of a depressive episode was significantly greater for patients on placebo (n=192) vs TRINTELLIX (n=204) during the first 24 weeks of the double-blind phase (HR, 2.01; 95% CI, 1.26-3.21; P<0.01). Half as many patients experienced recurrence (13% [n=27] vs 26% [n=50], P<0.01).
Study 9 open-label phase, AEs (incidence, ≥5%)12,14
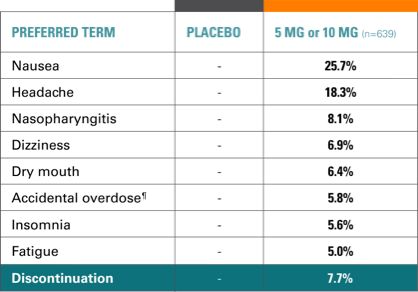
- 87% of AEs were mild to moderate
- 7.7% of patients withdrew due to AEs, most commonly: nausea, vomiting, headache, fatigue
- 2.2% of patients had SAEs, none occurring in >1 patient, except depression (2 patients)
- 1 patient withdrawn due to score ≥5 on MADRS item 10 (suicidal thoughts)
- 1 patient attempted suicide and was withdrawn; 1 patient had suicidal ideation but continued in the study; neither attempt nor ideation was related to TRINTELLIX.
Study 9 double-blind phase, AEs in at least 1 TRINTELLIX group (incidence, ≥5%)14
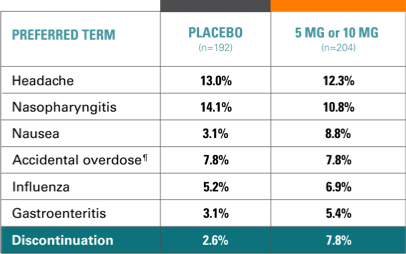
- In both groups, ~90% of AEs were mild to moderate4
- 2.6% (placebo) and 7.8% (TRINTELLIX) withdrew due to AEs
- 4 patients (placebo) and 7 patients (TRINTELLIX) reported SAEs; none occurred in >1 patient except road traffic accident (2 patients)
Note: One patient who was randomized to placebo in the double-blind period had an intentional overdose and alcohol poisoning in the post-dose period.¶
*The dose of TRINTELLIX was fixed during Weeks 8 to 12.1
‡Remission was defined as MADRS total score ≤10 at both Weeks 10 and 12.1
§Approximately 75% of patients were taking 10 mg/day.1
||Recurrence of a depressive episode was defined as MADRS total score ≥22 or lack of efficacy as judged by the investigator.1
¶An overdose was predefined as an amount, such as number of tablets, greater than what had been prescribed for that patient.14
Abbreviation: FAS, full analysis set.

Michele Novella, APRN‑BC
Fairfield University, Medical Director and Owner of Star Psychiatric Healthcare, LLC, Danbury, Connecticut
"I will emphasize that TRINTELLIX has safety data up to 64 weeks in hundreds of patients."
Michele Novella, APRN-BC, is the medical director and owner of Star Psychiatric Healthcare in Danbury, CT. She is a past member of Western Connecticut Health Network’s (WCHN) medical staff where she worked in the Behavioral Health Department including Crisis Intervention, Intensive Outpatient Program running groups and teaching Dialectical Behavioral Therapy (DBT). In the Outpatient setting, Michele held several critical incident debriefings following traumatic events and losses that were experienced by the staff there.
After graduating from Fairfield University, Michele worked as a Registered Nurse at Memorial Sloan-Kettering Cancer Center in New York City. Later, she worked at WCHN’s inpatient oncology unit before being asked to serve as Nurse Manager for the Outpatient Oncology & Chemotherapy Infusion Services at Associated Internists of Danbury. During that time, she received the prestigious American Cancer Society’s State‑Wide “Excellence in Oncology Nursing Award” in 1991.
Michele later pursued her Master’s Degree of Nursing from Yale University, graduating with Honors. She is Board Certified by the ANCC as both a Clinical Nurse Specialist in Adult Psychiatric Nursing, as well as a Psychiatric Nurse Practitioner.
She holds specialized training in DBT and cognitive behavioral therapy (CBT) and Critical Incident Stress Debriefing. She held a pivotal role in the aftermath of the Sandy Hook Shootings and helped to organize and run the crisis services offered to the public at Newtown’s Reed Intermediate School and treated several of the Newtown Police Department’s first responders. She has been asked to speak to several groups on post‑traumatic stress disorder and stress responses after trauma.
Ms Novella was paid as a consultant for Takeda and Lundbeck.
Patients experienced relief from symptoms as early as Week 2 with the full antidepressant effect not seen until Week 4 or later1
Study 5: time course of treatment response6
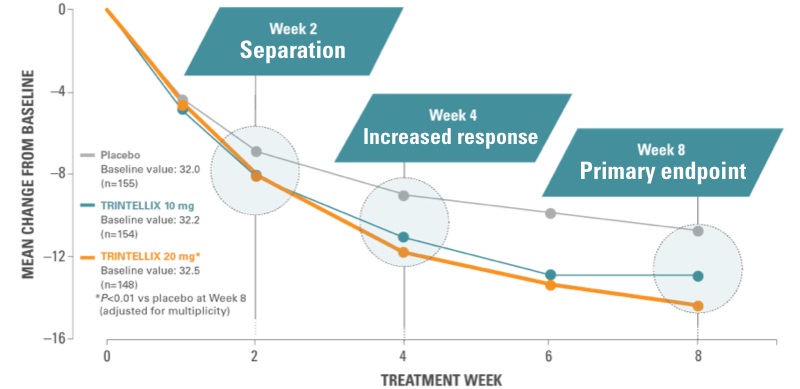
RELIEF AS EARLY AS WEEK 21
In the short-term studies, patients experienced relief from symptoms as early as Week 2, and the full antidepressant effect by Week 4 or later.
Results from a randomized, double‑blind, placebo-controlled, 8-week study (Study 5) of adult patients aged 18-75 years with a primary diagnosis of MDD (n=462).⁶ Chart shows mean reduction from baseline in MADRS total score at each visit (FAS, MMRM).
Based on the primary efficacy measure, the effect of TRINTELLIX was generally observed starting at Week 2 and increased over subsequent weeks, with the full antidepressant effect generally not seen until Week 4 or later.¹
*Doses significantly superior to placebo after adjusting for multiplicity
Abbreviations: MMRM, mixed model for repeated measures.
Learn more about TRINTELLIX

Support for
your patients
We’re here to help your eligible patients get the treatment you prescribe.

Offering
patients once
daily dosing
Learning about dosage amounts and drug interactions.

Resources for
you and your
patients
Find a range of information, education, and patient resources, as well as industry insights.